Generation of Monocyte Derived Dendritic Cells Using Xeno-free GMP Grade Human Platelet Lysate3/1/2017 
Human platelet lysate (hPL) is becoming an increasingly popular choice as a xeno-free serum supplement alternative to fetal bovine serum (FBS) for culture expansion of numerous other cell types. Our recent research focuses on the generation of monocyte derived dendritic cells using hPL instead of FBS or human AB serum (hABS).
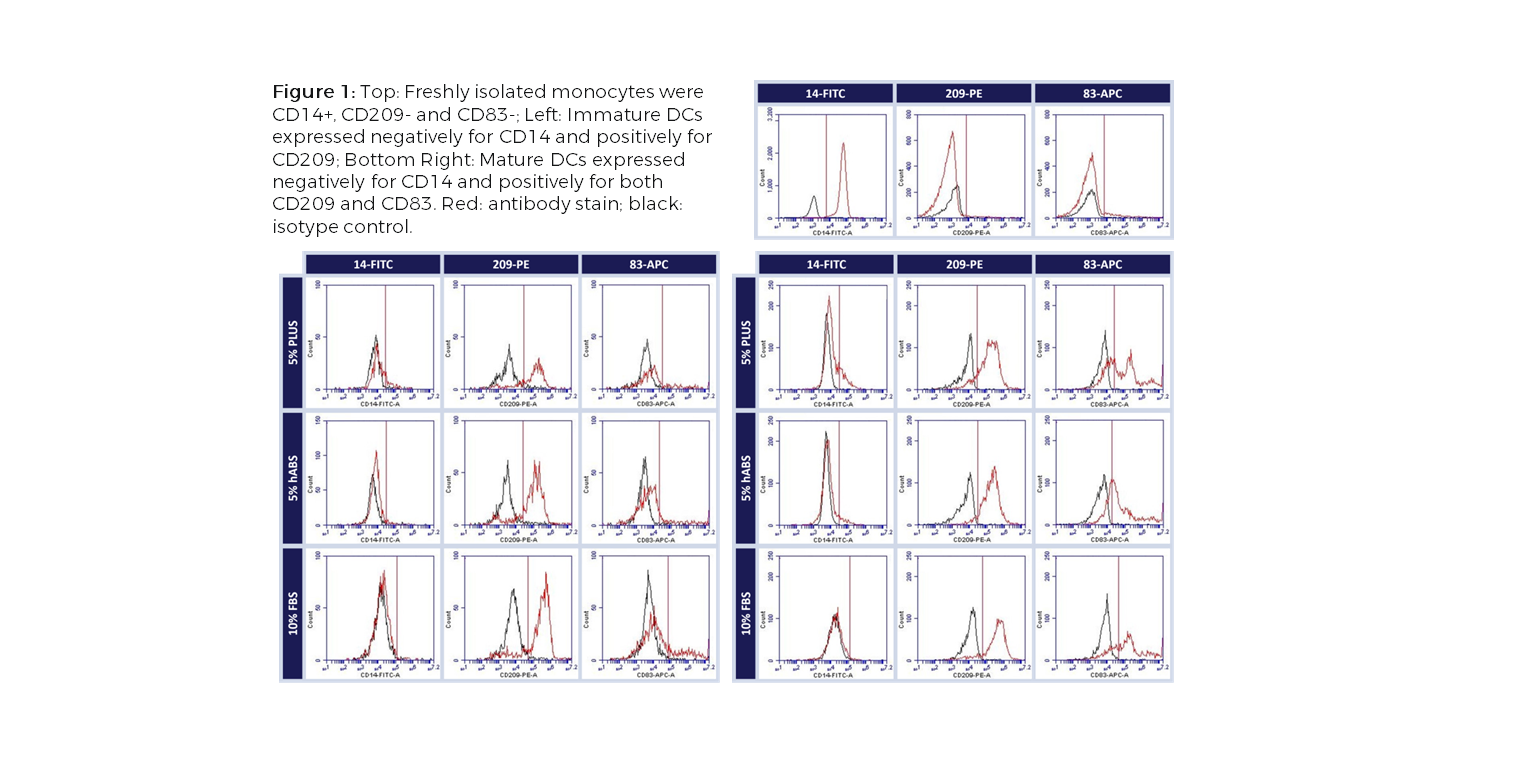
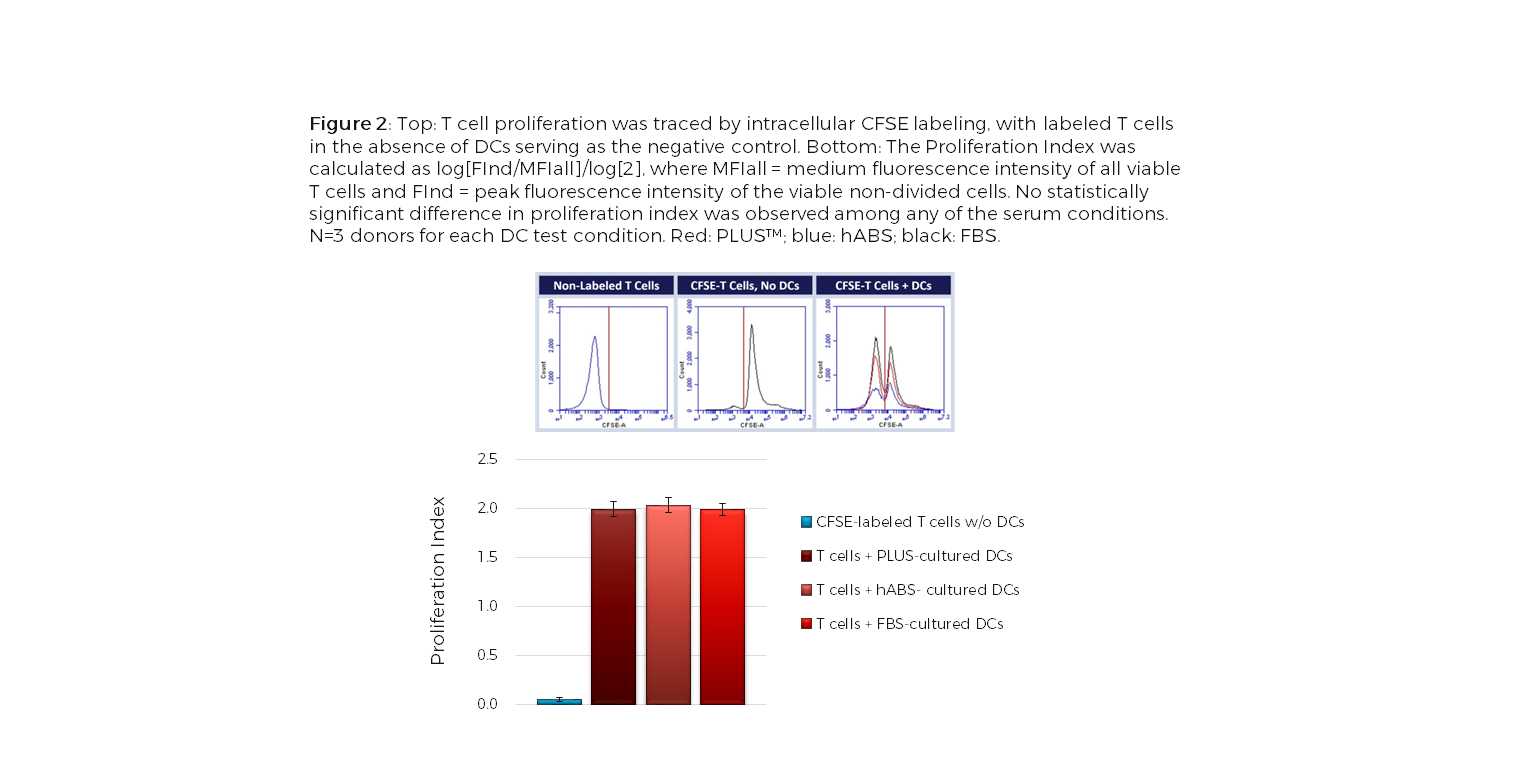
Dendritic cells (DCs), an important antigen-presenting cell type, have been widely used for therapeutic vaccine development in cancer immunotherapy. Among the different approaches to obtain DCs, ex vivo generation from peripheral blood isolated monocytes has been the most favorable method. The ex vivo differentiation of monocytes to immature DCs and their activation to mature DCs require addition of a serum supplement to the culture media. Limitations with Current Choice of Serum: Traditionally, clinical manufacturing of Mo-DCs has employed FBS or human AB serum (hABS). The use of FBS in cell therapy processes poses potential risks for viral and prion transmission as well as adverse immunological reactions while hABS which is collected from a small number of donors has considerable lot-to-lot inconsistency. These variations can significantly impact cell growth, morphology, and functionality, which is of concern with DCs because of their diverse phenotypic range. To address these issues, Compass Biomedical’s PLUS™ human platelet lysate was tested as a replacement for FBS and hABS in culture medium for differentiation and maturation of DCs from peripheral blood isolated monocytes. PLUS™ human platelet lysate is manufactured using a GMP production process with large lots of platelet units obtained from AABB-accredited blood banks, making it a more reliable alternative to hABS and a xeno-free alternative to FBS. Results Flow cytometry analysis of immature and mature Mo-DCs cultured in either PLUS™, hABS or FBS showed that PLUS™ produced mature DCs that were functionally equivalent to those cultured in FBS and hABS (CD14-, CD209+, CD83+, CD86+ and HLA-DR+), independent of the maturation pathway (TNF-α, IFN-γ or LPS) (Figure 1). A functional co-culture assay using PLUS™-cultured mature DCs showed that they stimulated allogeneic T cell proliferation in vitro as efficiently as Mo-DCs cultured in other serum (Figure2). Together, the results show that PLUS™ human platelet lysate can successfully replace FBS or hABS for large-scale ex vivo production of clinical grade Mo-DCs for immunotherapy trials. For further experimental details and results, check out our poster presented at the 2016 ISCT North America conference.
1 Comment
|
|
Compass Biomedical 45 South Street Hopkinton, MA 01748, USA Email: info@compassbiomed.com Phone: 508-283-2622 |
Copyright© 2020 Compass Biomedical, Inc. All rights reserved.